- info@bpendo.org
- (405)-974-0776
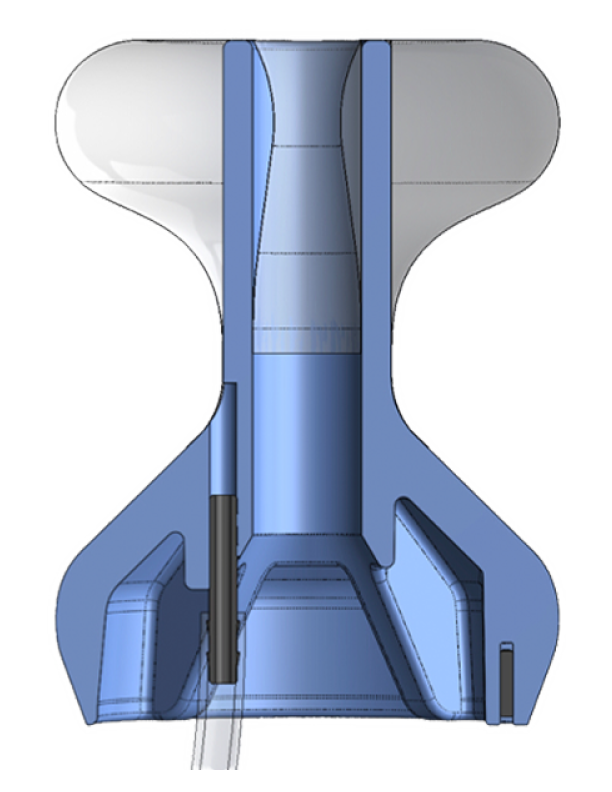
The IRD is an add-on device, intended to make colonoscopies safer and more efficient.
*The IRD is FDA approved.
Insufflation of the colon is required for visualization during colonoscopy. However, when air or fluid incontinence occurs, maneuverability and visualization are compromised. This issue costs valuable time, adds frustration for the physician and staff, may result in sub-optimal exam, earlier repeat colonoscopies, and increased costs.
BPEndo is proud to announce we recently closed a $3M Series A round for scaling manufacturing and commercialization. Our hope is to launch product in Q1 2025
“In summary, patients with multiple comorbidity and patient who require a longer procedure are statistically more likely to suffer from air incontinence. Additionally, using MAC with Propofol over traditional conscious sedation is a confounding factor.” – Dr. Madhoun



BPEndo is led by experienced leaders in the medical device industry. The diverse background of the team puts the company in a strong position to create value going forward.



